Computational Modeling in f-element Chemistry
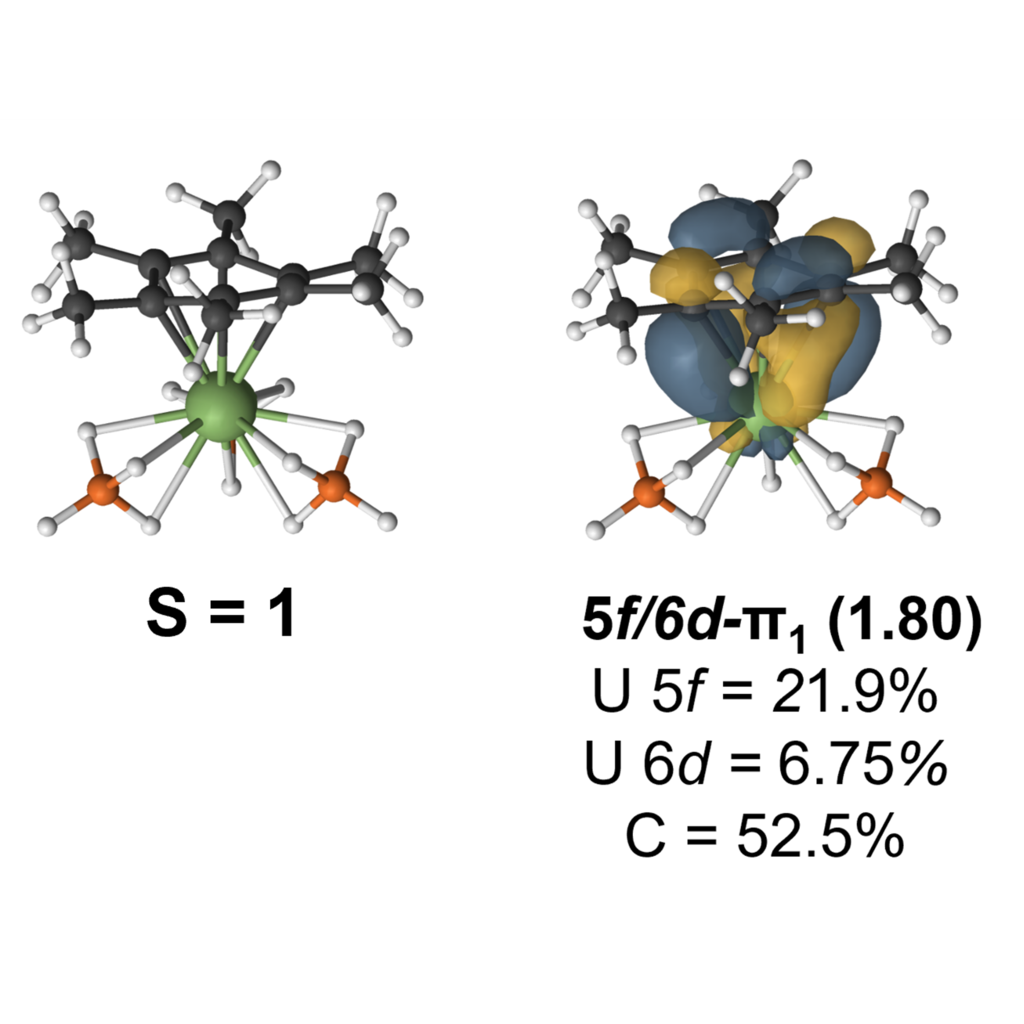
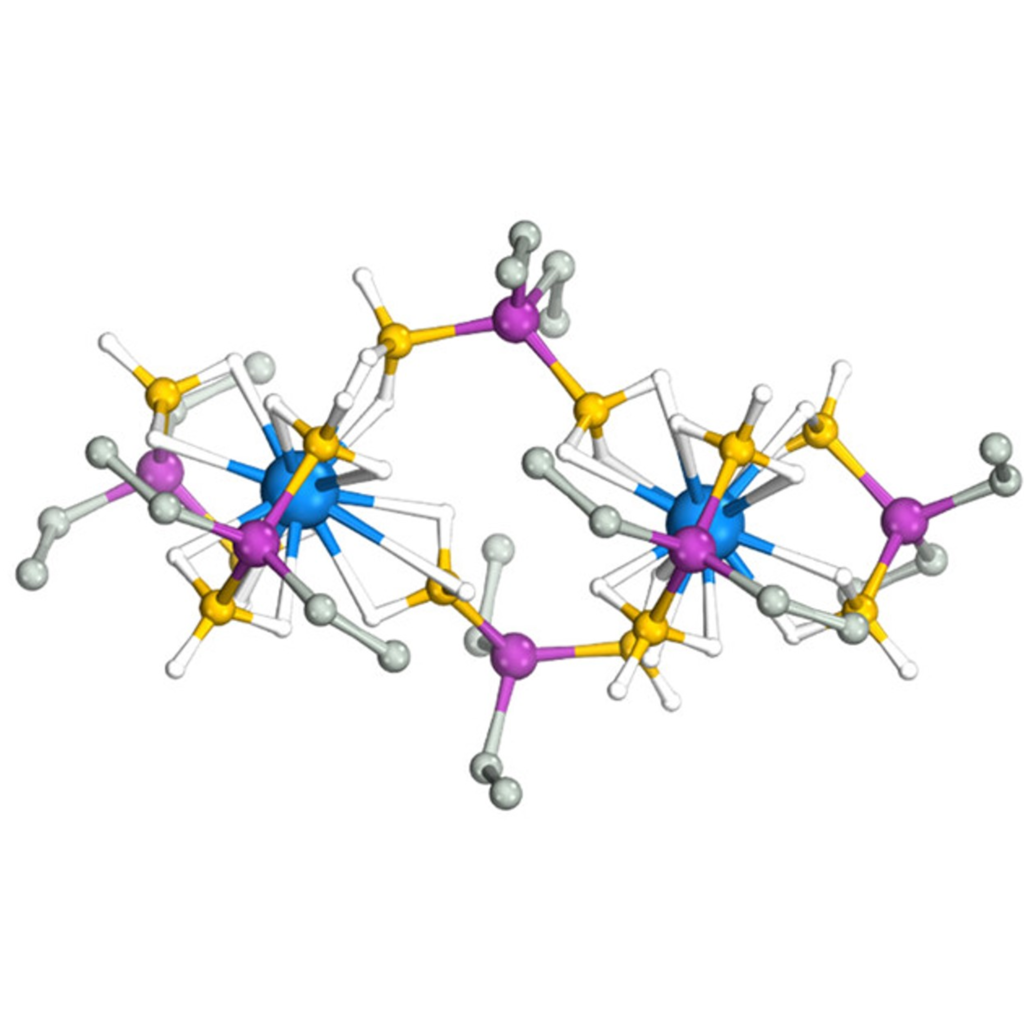
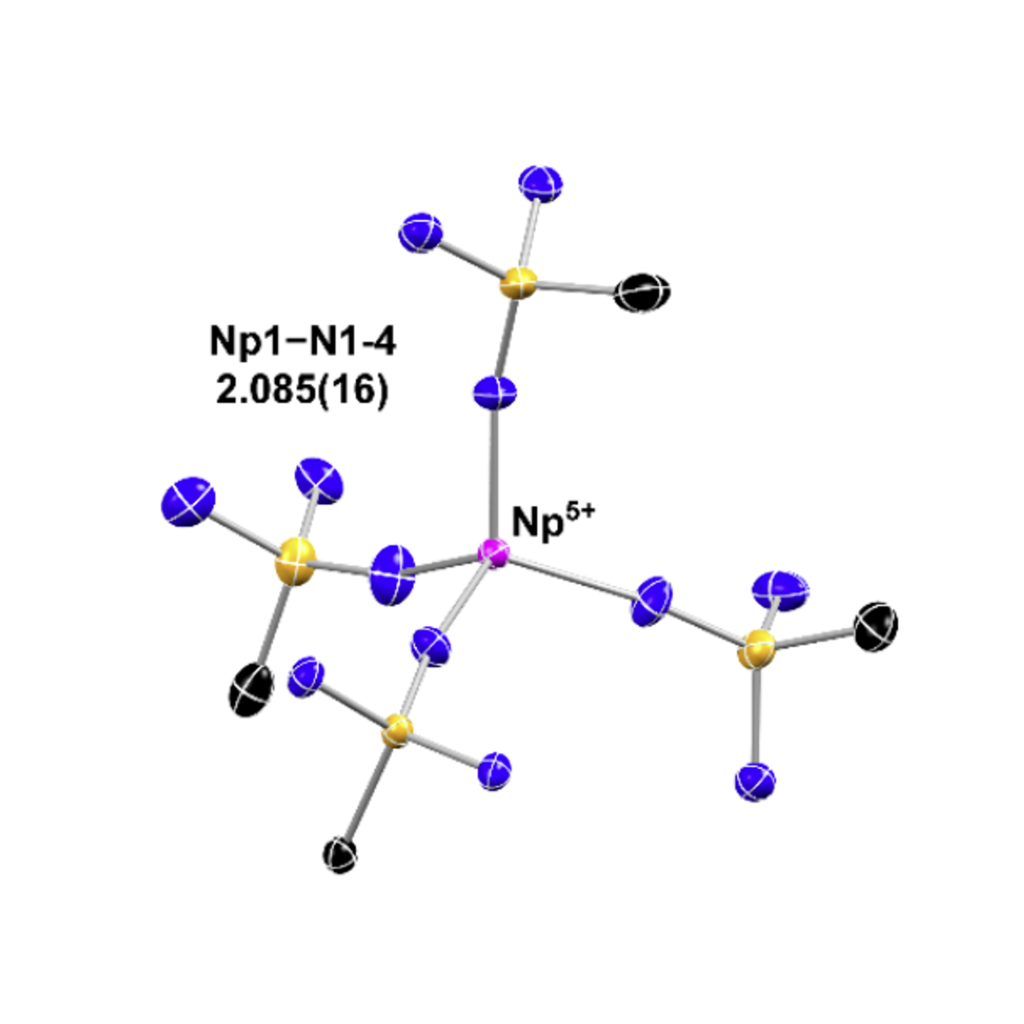
The 5f-orbitals and 6d-orbitals are well-known to engage in bonding motifs with multiconfigurational electronic structures and spin-orbit effects are frequently large. We perform state-of-the-art calculations that can be systematically improved on systems ranging from small molecules to large coordination complexes containing actinides. Our goal is to use higher-level methods than traditionally applied in the literature. We make synergistic comparisons between theory and highly accurate spectroscopic measurements. By computing properties using fully relativistic CASPT2 methods based on the Dirac equation (Dirac-CASPT2), we can understand the importance of the different relativistic effects as well as characterize the contributions from non-dynamic and dynamic correlation. Specifically, we study organoactinide complexes in collaboration with synthetic chemists, small molecules in collaboration with spectroscopists, and examine systematically chosen systems in collaboration with theoretical chemists developing new methods.
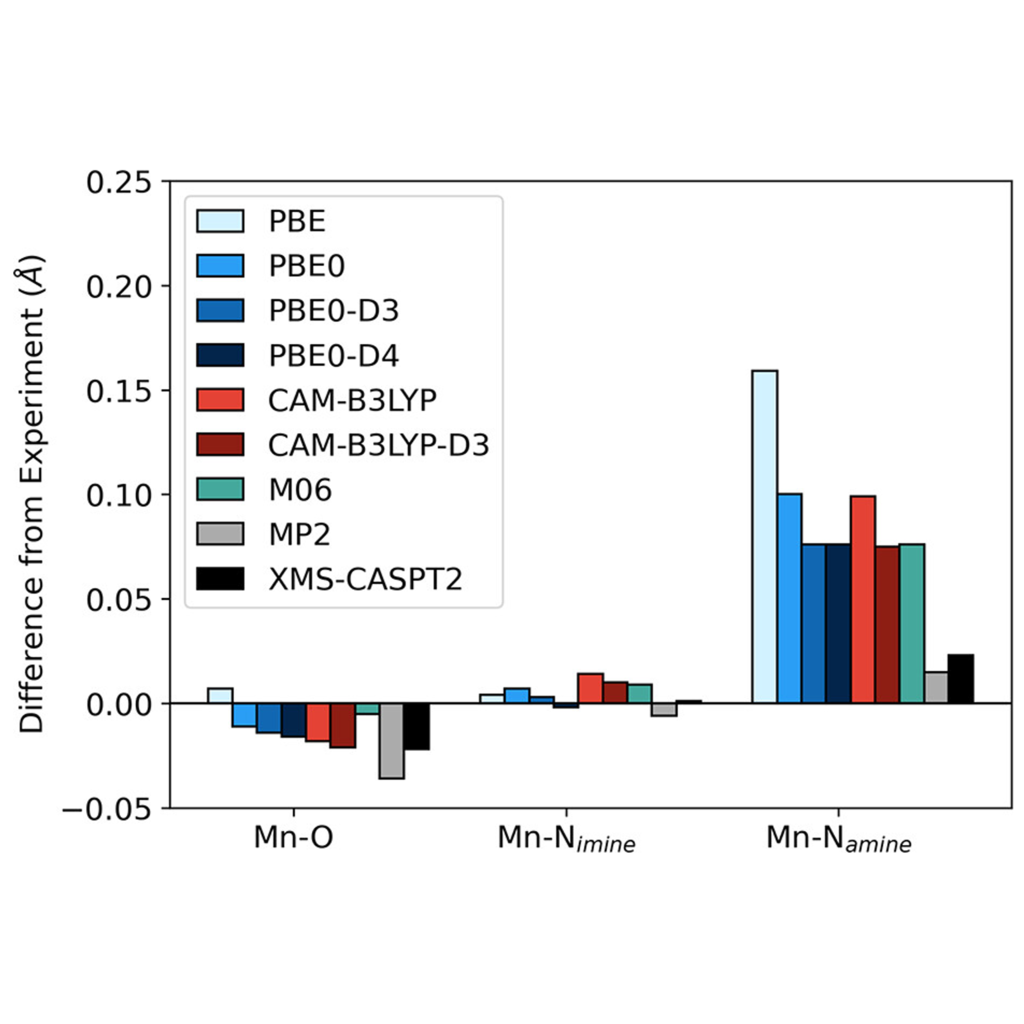
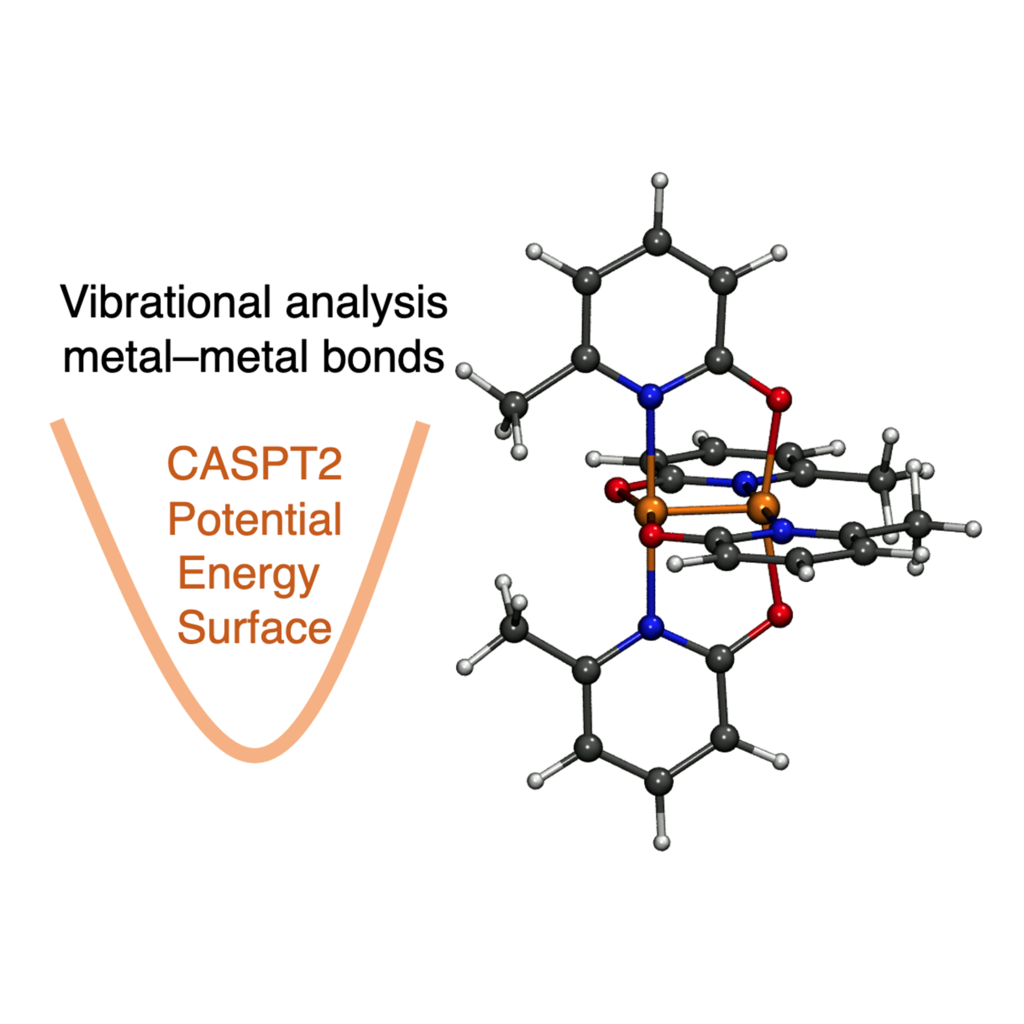
CASPT2 Molecular Geometries in Transition Metal Chemistry
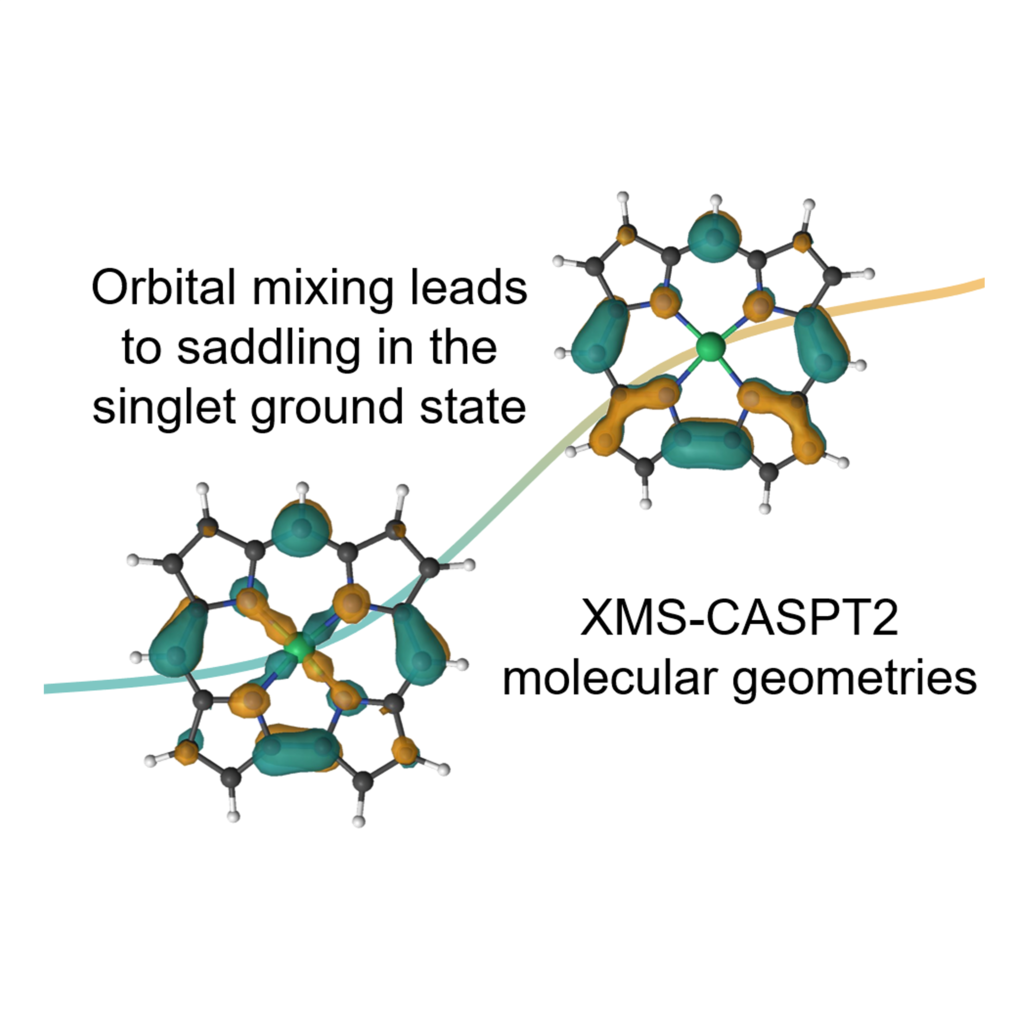
Recent technological development of fully internally contracted gradients for second-order multireference complete active space methods (CASPT2) has enabled the geometry optimization of medium to large transition metal complexes. Our group has focused on using these methods to model the molecular geometries of iron spin-crossover complexes, copper corrole complexes, and Cr–Cr multiple bonds with CASPT2. While DFT geometries are often quite good for minima, this can break down when the electronic structure is best described with a multireference method. By performing geometry optimizations for the correct multireference ground state, one can ensure that the potential energy surface is described appropriately and avoid needing to take care with functional choice. This work is facilitated by implementations in the BAGEL program package, of which we are developers.
Contributing Fundamental Understanding to Impact Global Challenges
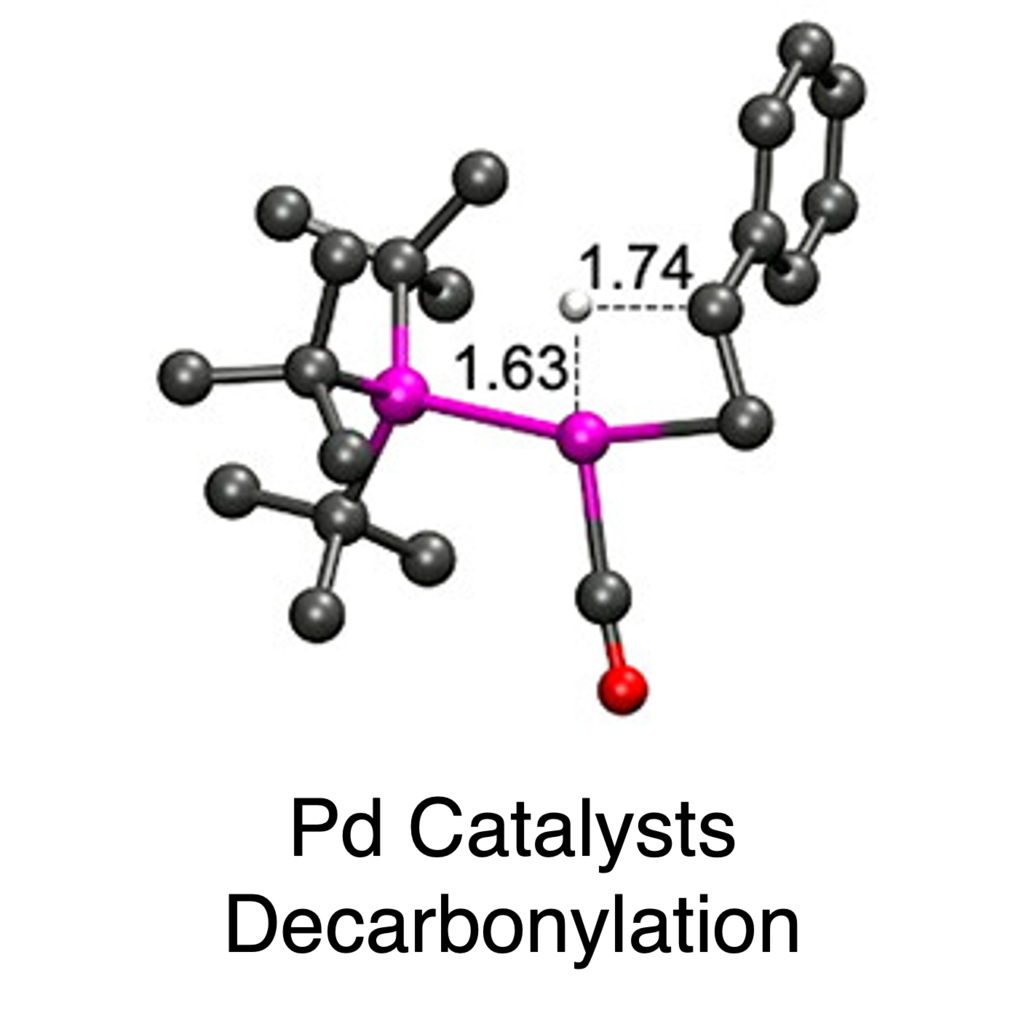
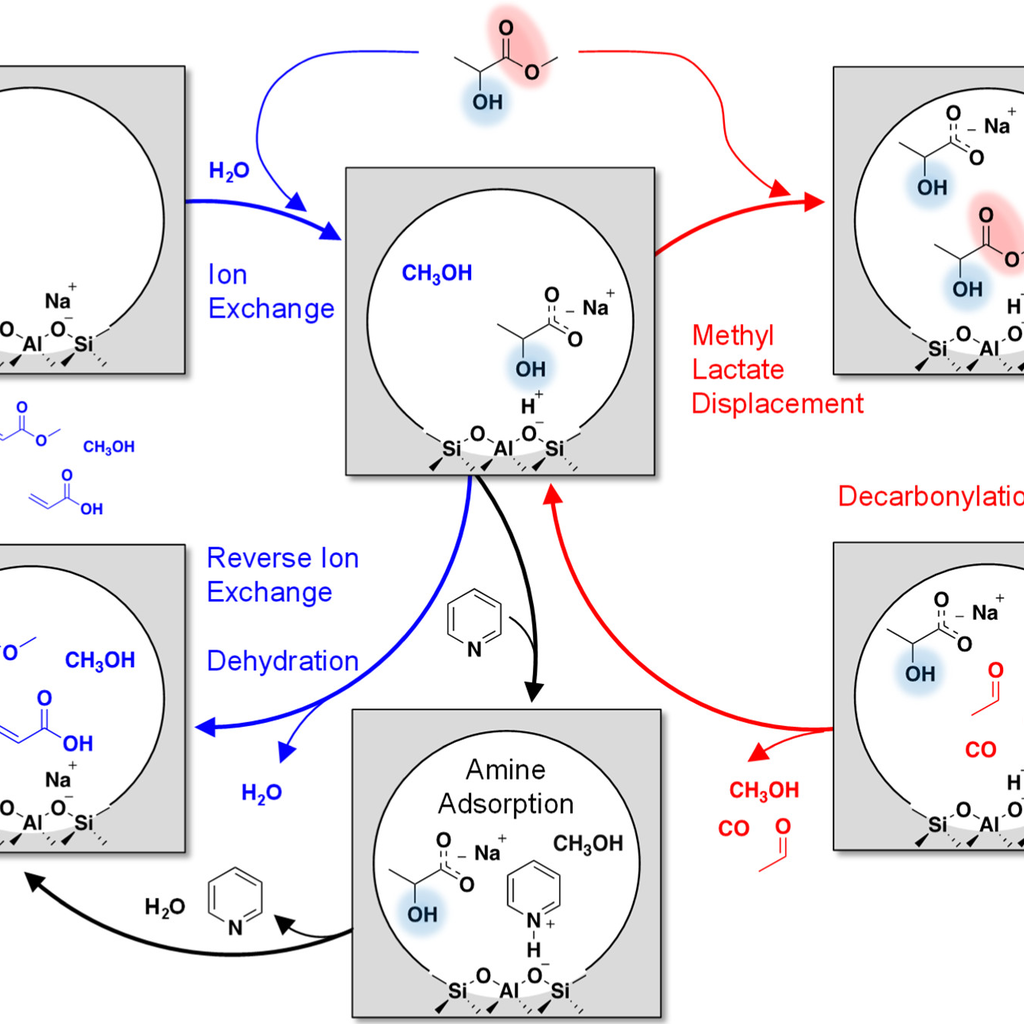
Our group is motivated by a strong desire to contribute to large scall challenges in the environment. These problems cannot be solved alone and are often addressed in large collaborations. Within the NSF Center for Sustainable Polymers (NSF CSP), we perform computational modeling in combination with experiment to provide molecular-level insights to reaction mechanisms in order to guide the design of the next generation of catalysts. By means of a combination of density functional theory (DFT) and classical simulations, we address questions arising from experiments performed in the Center. We are currently collaboration with the Tolman group at Washington University in St. Louis, the Dauenhauer and Tonks groups at the University of Minnesota, and the Kalow group at Northwestern University. We are also members of the Environmentally Applied Refrigerant Technology Hub (EARTH), an NSF Engineering Research Center. EARTH is founded on four pillars: convergent research, diversity and culture of inclusion, engineering workforce development, and innovation ecosystem, which will impact the engineering and scientific communities, the HVACR industry, and society. Our role is to use quantum chemical methods for designing catalysts to make new refrigerants, to design novel sensors, and to improve separations.